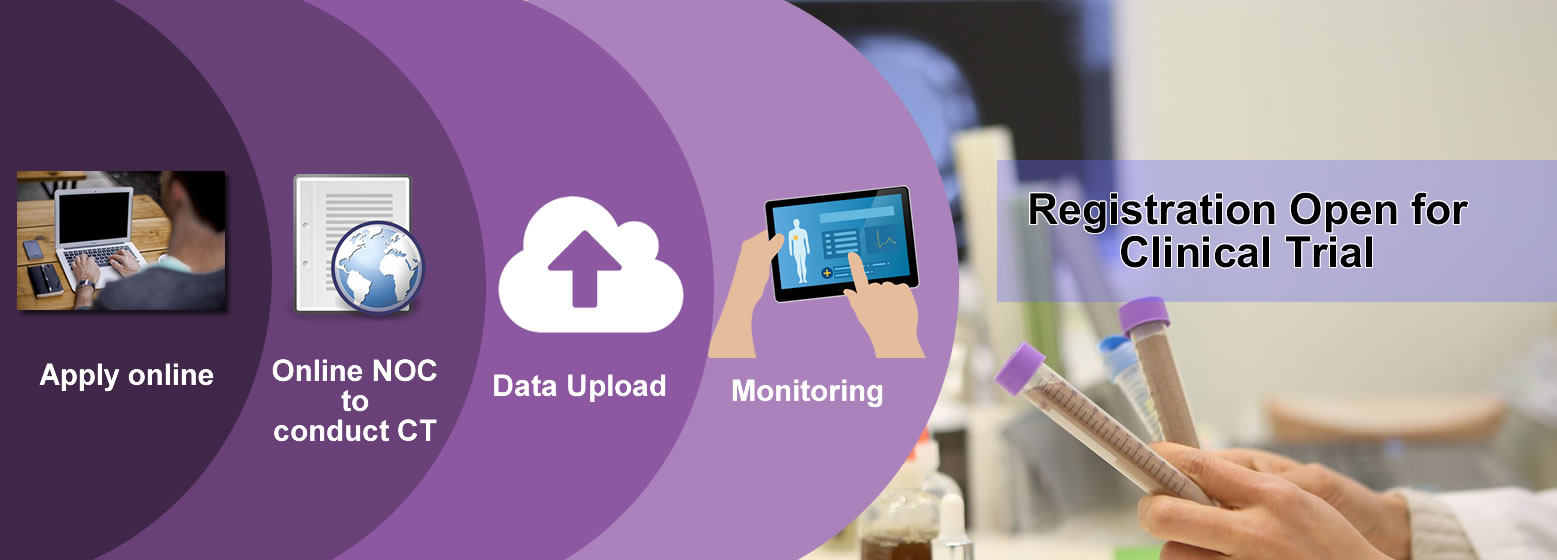
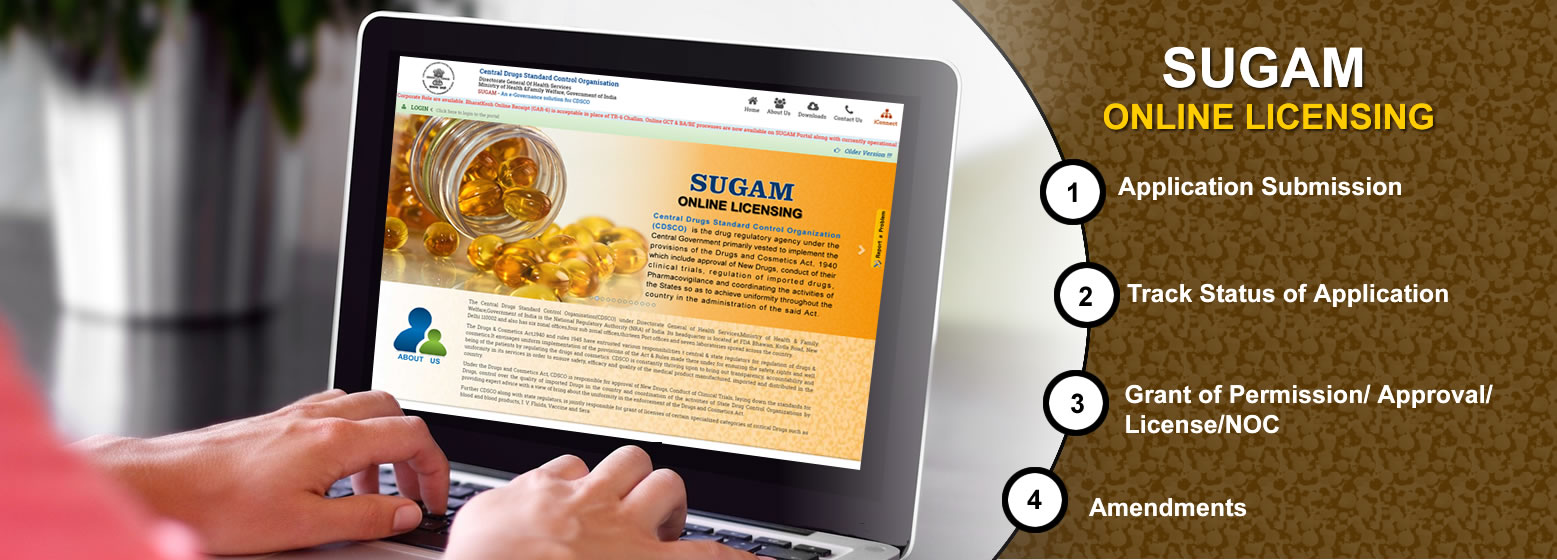
Applicants can apply online on
SUGAM Portal for Permission to Manufacture , Import or to conduct
Clinical Trials as per new Clinical Trial rules of Drugs & Cosmetics
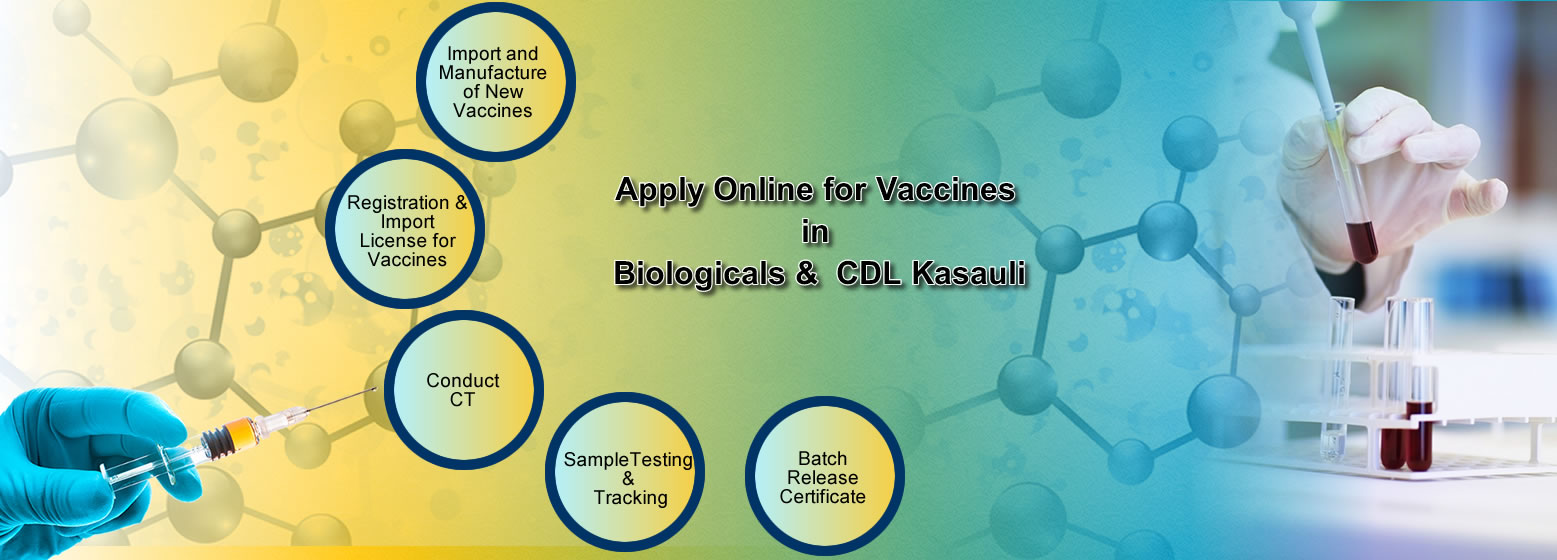
Act. Online process for Biologicals(Vaccines & r-DNA) is available
on SUGAM. Manufacturers can add their Formulations Data on SUGAM
Portal. New drugs approved by CDSCO is published under drugs@ CDSCO
section. Firms can request to test vaccine samples at CDL Kasauli
and receive batch release certificate online. Firms can add their
License details issued by State FDA's on SUGAM.
LOGIN/SIGN UP Click here to login/sign up to the portal
 Step-2 Export NOC Application for Port Office NOC is live now.(User Manual For Export NOC(Port))
Step-2 Export NOC Application for Port Office NOC is live now.(User Manual For Export NOC(Port))
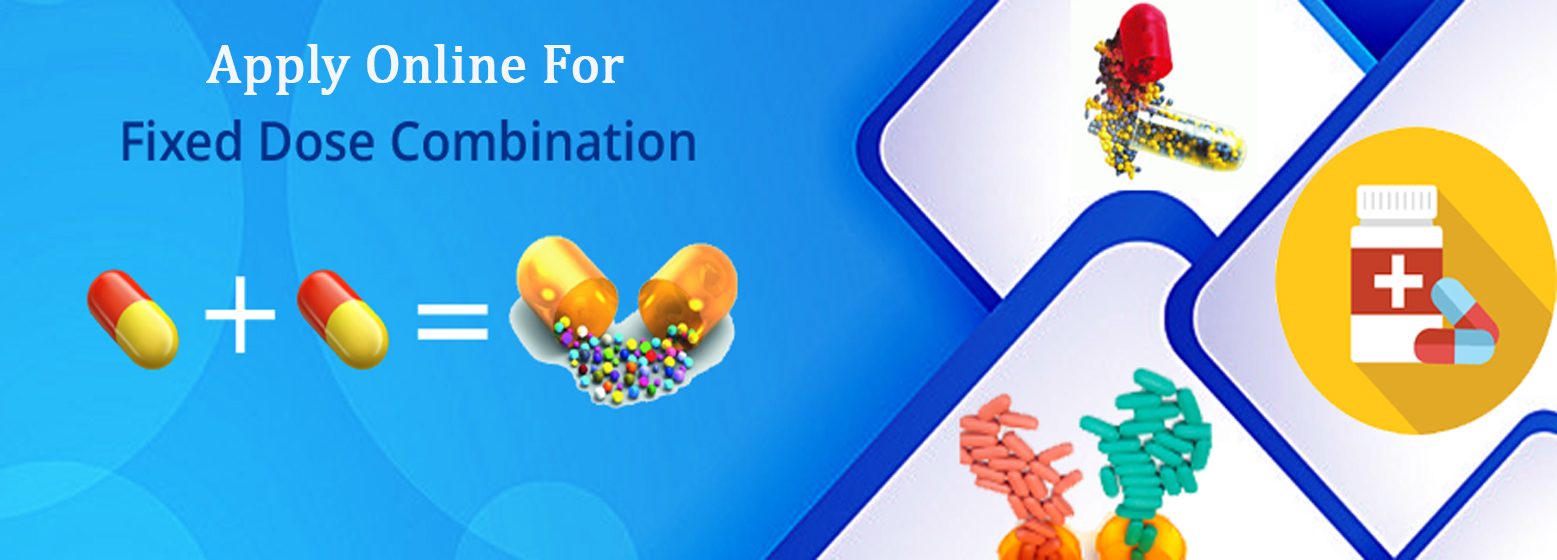
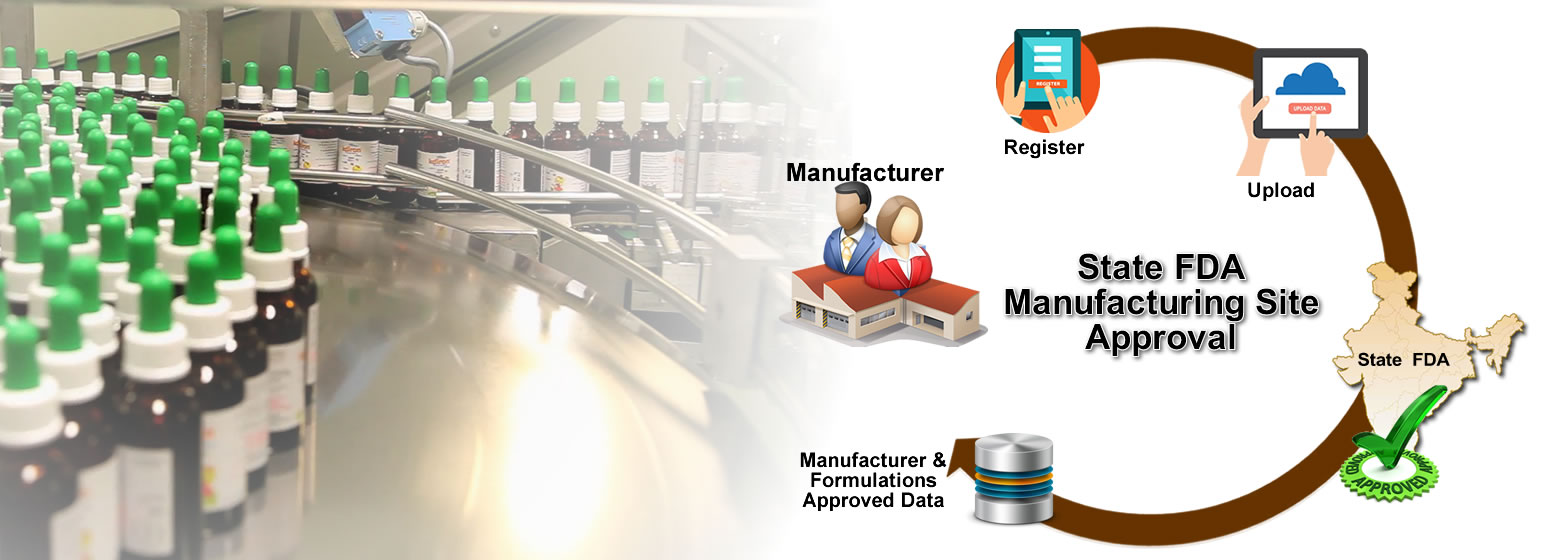
Register Data with State FDA
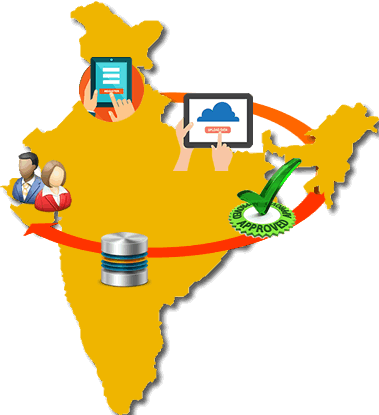
Sugam builds a comprehensive database of the various permissions and licenses issued by State FDAs. This includes details of manufacturers, manufacturing site and drug formulations. Manufacturer can view their consolidated data about permissions issued to them from State FDA.
Important Links
PORTAL STATISTICS
Learn about number of applications submitted on SUGAM portalPATIENTS
Learn about drug/device approvals
DRUGS @ CDSCO
Approved Drugs by CDSCOFEATURES



Our Prcess
Online Submission
Review
Grant of NOC/ Permission
Gallery











Contact Us
ADDRESS
Central Drugs Standard Control OrganizationDirectorate General of Health Services,
Ministry of Health and Family Welfare,Government of India
FDA Bhavan, ITO, Kotla Road, New Delhi -110002
Online Help
P : 011-23502918(CDSCO)F : 91-11-23236973
Assistance
IT Related : ithelpdesk.sugam[at]cdsco[dot]nic[dot]in: helpdesk[dot]sugam[at]cdsco[dot]nic[dot]in